Understanding how to calculate relative atomic mass is a key skill in Chemistry, especially for GCSE, IGCSE, and IB students. Many learners memorise the formula but struggle to apply it correctly in exams.
The good news is this topic is much easier than it looks—once you truly understand what relative atomic mass means and why isotopes matter.
Relative atomic mass (often written as Ar) is the average mass of an element’s atoms, taking into account the different isotopes and how common they are.
It is:
A weighted average
Based on carbon-12 as the standard
Found on the Periodic Table
👉 Important point:
Relative atomic mass is not a whole number for most elements.
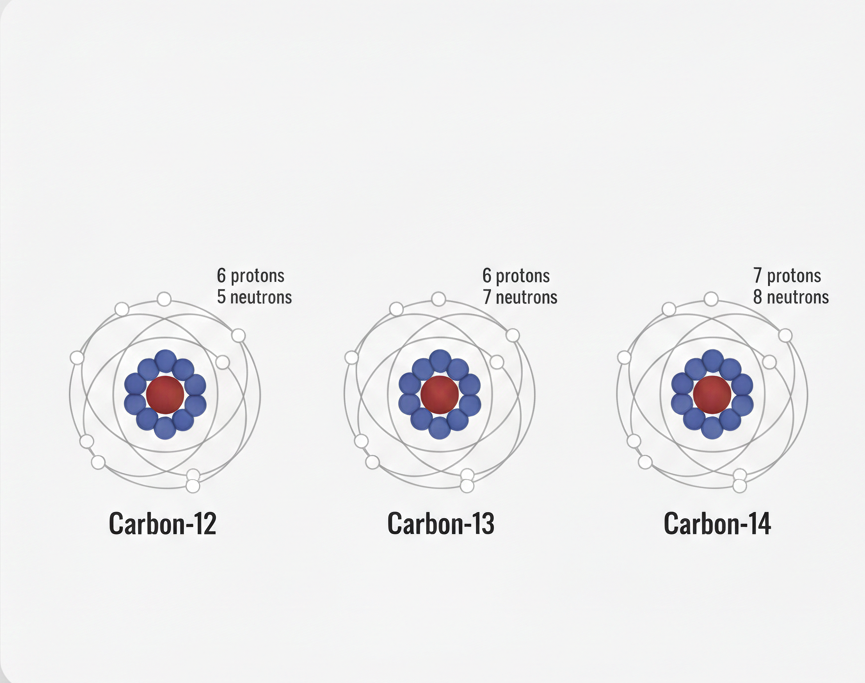
The word relative means we are comparing masses.
Relative atomic mass compares the mass of an atom to:
1/12th of the mass of a carbon-12 atom
This is why carbon-12 is the reference standard used worldwide.
Now let’s look at the most important part.

In words:
Multiply each isotope’s mass by its percentage abundance, add them together, then divide by 100.
Example 1: Chlorine
Chlorine has two isotopes:
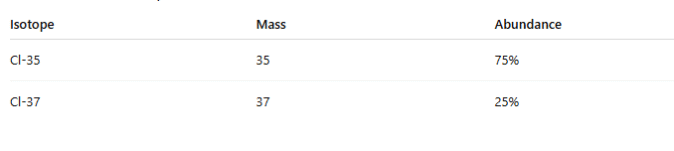
Step 1: Multiply mass × abundance
35 × 75 = 2625
37 × 25 = 925
Step 2: Add results
2625 + 925 = 3550
Step 3: Divide by 100
3550 ÷ 100 = 35.5
✅ Relative atomic mass of chlorine = 35.5
Many students incorrectly do this:
(35+37)÷2=36❌
This is wrong because:
Isotopes are not equally abundant
One isotope exists more than the other
Examiners look for weighted calculations, not guesses.
| Isotope | Mass | Abundance |
|---|---|---|
| Mg-24 | 24 | 79% |
| Mg-25 | 25 | 10% |
| Mg-26 | 26 | 11% |
(24 × 79) = 1896
(25 × 10) = 250
(26 × 11) = 286
Total = 2432
2432 ÷ 100 = 24.32
✅ Relative atomic mass of magnesium = 24.3 (rounded)
Scientists determine isotopic abundance using mass spectrometry.
A mass spectrometer:
Separates isotopes
Measures their mass
Calculates abundance
You don’t need to memorise the machine details for GCSE, but you must understand the output.
Many students confuse these two.
| Term | Meaning |
|---|---|
| Mass number | Protons + neutrons (whole number) |
| Relative atomic mass | Weighted average of isotopes |
Relative atomic mass is not for a single atom — it’s for the element as a whole.
You may be asked to:
Calculate relative atomic mass
Explain why it’s not whole
Use Ar values in further calculations
Interpret isotope data
This topic links directly to:
Relative formula mass (Mr)
Mole calculations
Chemical equations
Always divide after multiplying by abundance.
Check abundance values carefully.
Round only at the final step.
An element X has two isotopes:
| Isotope | Mass | Abundance |
|---|---|---|
| X-10 | 10 | 60% |
| X-11 | 11 | 40% |
Answer:
(10 × 60) + (11 × 40) = 600 + 440 = 1040
1040 ÷ 100 = 10.4
It is the average mass of an element’s atoms, considering all its isotopes.
Because isotopes exist in different proportions.
No. Exam questions always provide abundance data.
No. Mass number is for one atom; relative atomic mass is an average.
Because it is stable and easy to measure accurately.
Yes. It links to moles, stoichiometry, and exam calculations.

IB Demystified is a trusted online learning platform led by certified IB examiners and educators.
© 2026 IB Demystified LTD
ALL RIGHTS RESERVED.
Powered by AfiaDigital
WhatsApp us